Atomic Isotopes
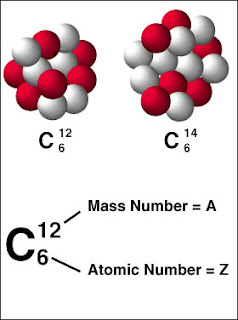
A major characteristic of an atom is its atomic number, which is defined as the number of protons. The chemical properties of an atom are determined by its atomic number and is denoted by the symbol Z. The total number of nucleons (protons and neutrons) in an atom is the atomic mass number. This value is denoted by the symbol A. The number of neutrons in an atom is denoted by N. Thus the mass of an atom is A = N+Z.
Nuclear Isotopes
Atoms with the same atomic number but with different atomic masses are called isotopes. Isotopes have identical chemical properties, yet have very different nuclear properties. For example, there are three isotopes of hydrogen. Two of these isotopes are stable, (not radioactive), but tritium (one proton and two neutrons) is unstable. Most elements have stable isotopes. Radioactive isotopes can also be created for many elements.
Atoms with the same atomic number but with different atomic masses are called isotopes. Isotopes have identical chemical properties, yet have very different nuclear properties. For example, there are three isotopes of hydrogen. Two of these isotopes are stable, (not radioactive), but tritium (one proton and two neutrons) is unstable. Most elements have stable isotopes. Radioactive isotopes can also be created for many elements.

No comments:
Post a Comment